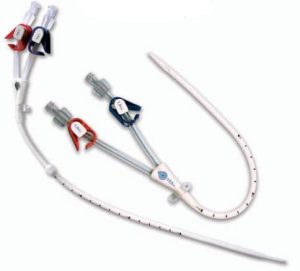
The Sirolimus-eluting coronary stent is made from a cobalt chromium alloy called L605, coated with a mix of sirolimus and last generation of biostable polymers. The stent is pre-mounted on the delivery system that will allow implantation on the lesion to treat thanks to the inflation of a balloon at the distal end of the catheter. The stent has an open cell design and the delivery system is a rapid exchange balloon catheter, having a single lumen configuration on the proximal part and a coaxial double lumen configuration on the distal part. There are radiopaque markers delimiting the length of the stent and to facilitate observation under fluoroscopy. The distal part of the catheter is coated with a durable hydrophilic coating to minimize friction and improve its trackability. The inflatable segment is coated with a homogeneous mixture of the drug Sirolimus (Rapamycin) and a biostable fluoroacrylate polymer yielding a drug dose of 1.4 µg/mm2.
The stent is indicated for:
- Patients with symptomatic ischemic heart disease due to “de novo” stenotic and re-stenotic lesions located in the coronary arteries.
- Patients with occlusive disease due to acute myocardial infarction located in in the coronary arteries.
Diameter from 2 to 4.5 mm and lengths from 9 to 49 mm. Largest overexpansion currently on the market: Lengths: 2, 2.25, 2.50 expand to 4mm, Lengths: 2.75, 3, 3.50 expand to 5.25mm, Lengths: 4 and 4.50 expand to 6mm.